Extremely early genomic events and temporal order of esophageal squamous cell carcinogenesis: Longitudinal self-comparison of progressors and non-progressors
Abstract
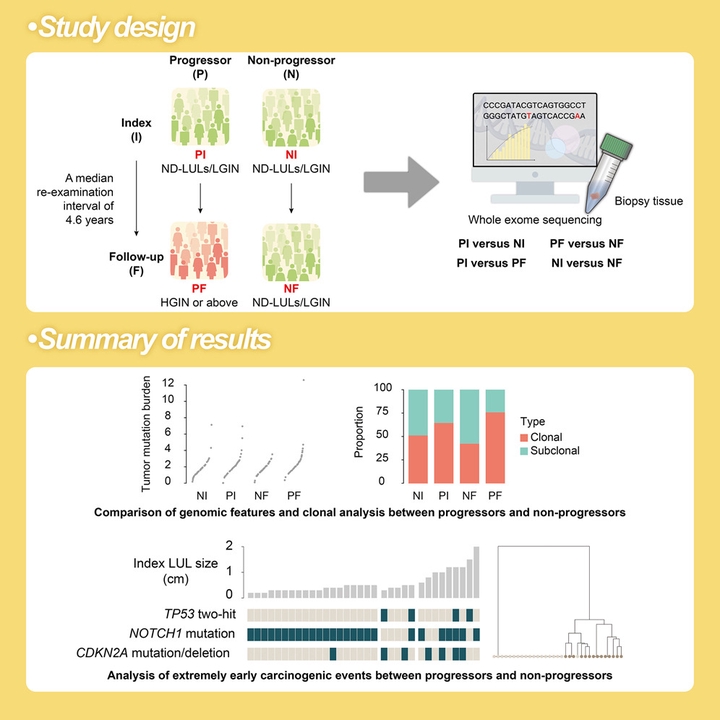
The current surveillance guideline of esophageal squamous cell carcinoma (ESCC) runs the risk of underestimation of early Lugol-unstained lesions (LULs), and extremely early genomic events in the carcinogenesis and their temporal order of occurrence remain unclear. Here, we performed whole-exome sequencing analyses of 148 biopsy samples obtained at different time points (with a median 4.6-year interval) from the same esophageal lesions of 74 asymptomatic subjects with LULs detected at community-based screening, of whom 33 individuals showed progression at the follow-up chromoendoscopy, while the other 41 did not. We found that progressors showed higher tumor mutational burden, chromosomal instability level, whole-genome doubling (WGD) events, and apolipoprotein B mRNA-editing catalytic polypeptide-like (APOBEC) activity at both index and follow-up compared to non-progressors. Sustained TP53 two-hit events, absence of NOTCH1 mutation, presence of CDKN2A mutation/deletion, and WGD were detected both before and after LUL progression in 64% (9⁄14) of progressors and none (0/19) of non-progressors with non-dysplastic LULs (ND-LULs). CCND1, FGFs, and MIR548K amplification in chromosome 11q13.3 only occurred in progressors with high-grade intraepithelial neoplasia or above lesions. TP53 two-hit events, absence of NOTCH1 mutation, and presence of CDKN2A mutation/deletion were positively correlated with WGD and successfully distinguished all 5 progressed individuals from the 24 subjects at so-called “low risk” of progression (ND-LULs with a size of ≤5 mm) under current surveillance criteria. Collectively, TP53 two-hit events, absence of NOTCH1 mutation, and presence of CDKN2A mutation/deletion are extremely early events in the carcinogenesis of ESCC, providing early warning markers for the surveillance of high-risk precursor lesions of ESCC.